DC-STIMULATOR MOBILE
Portable tDCS device for clincial practice and home-based care
Straight to:

The DC-STIMULATOR MOBILE is a clinical simulator designed to be used in clinics and practices. Using this device, doctors and psychologists can apply transcranial direct current stimulation (tDCS) using weak currents up to 2mA over 15-30 minutes.
tDCS represents part of interventional neurophysiology; the electrical charges and densities administered during tDCS lie far below the threshold for releasing a stimulus and have a modular effect on existing neuronal elements. Depending on the duration, used current, current density and frequency, the stimulation is effective on inhibiting or activating cortical activity.
The DC-STIMULATOR MOBILE is used for transcranial Direct Current Stimulation for symptom relief in depression.




Applications of the DC-STIMULATOR MOBILE
The DC-STIMULATOR MOBILE can be used as part of the day-to-day routine of therapy centers and practices.
This device is best used when:
- Therapy is carried out according to a fixed protocol, which isn’t changed
- Patients are to be treated within fixed time spans.
When ordering the device, the user specifies a stimulation configuration. A configuration can include up to 8 different stimulation sequences, which can then be selected on the stimulator unit. After the device is delivered, the DC-STIMULATOR MOBILE only functions in this mode. This effectively prevents inadvertent or unintended changes to the stimulation parameters and makes the day-to-day usage of the device easier.
If you intend to use the DC-STIMULATOR MOBILE in double blind clinical studies applying transcranial electrical stimulation (tDCS, tACS, rTMS) please contact the manufacturer or responsible sales department.
In clinical research the devices are used by the German Center for Brainstimulation as well as the Charité in Berlin, the LMU in Munich, the University hospital Hamburg-Eppendorf in Germtes worldwide.
"By now, tDCS has become an integral part of our therapeutic approach due to its good efficacy and simple handling. It (...) can be very well integrated into the clinical routine of a psychiatric-psychotherapeutic practice"
Frank Schmidt-Staub - Neurologist and Psychiatrist in Hannover, Germany

Features of the DC-STIMULATOR MOBILE
The DC-STIMULATOR MOBILE unifies easy usage with exceptional patient safety.
The path of the electrical current is constantly monitored by both hardware and software. The continuous monitoring of electrode impedance allows insufficient skin contact to be detected, leading to the stimulation being automatically cut off. Injuries to the patient are thus reliably avoided. For the first time, the DC-STIMULATOR MOBILE makes it possible to analyse the stimulation data recorded by the device after a therapy session.
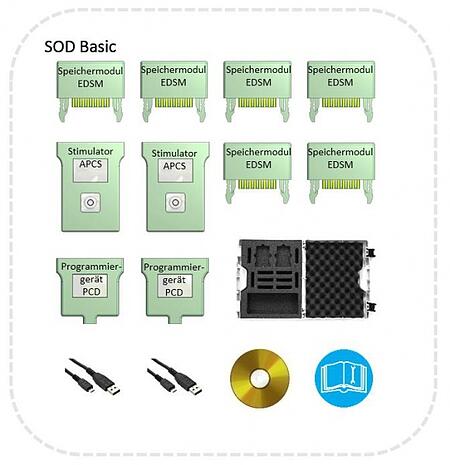
The DC-STIMULATOR MOBILE is delivered as a so-called Set of Devices (SOD). The basic package (SOD BASIC) can be supplemented as needed for different therapy situations.
The components of the SOD Basic have been designed to allow the user to work as comfortably as possible:
- Two treatments can be carried out at the same time.
- Concurrently to a treatment, two storage modules can be prepared and charged. As a result, waiting times between treatments are avoided, which would otherwise be necessary for the charging process.
- Two further storage modules are kept as a reserve.
- At least one replacement device is automatically kept available in case of a defect.
Components of the SOD BASIC
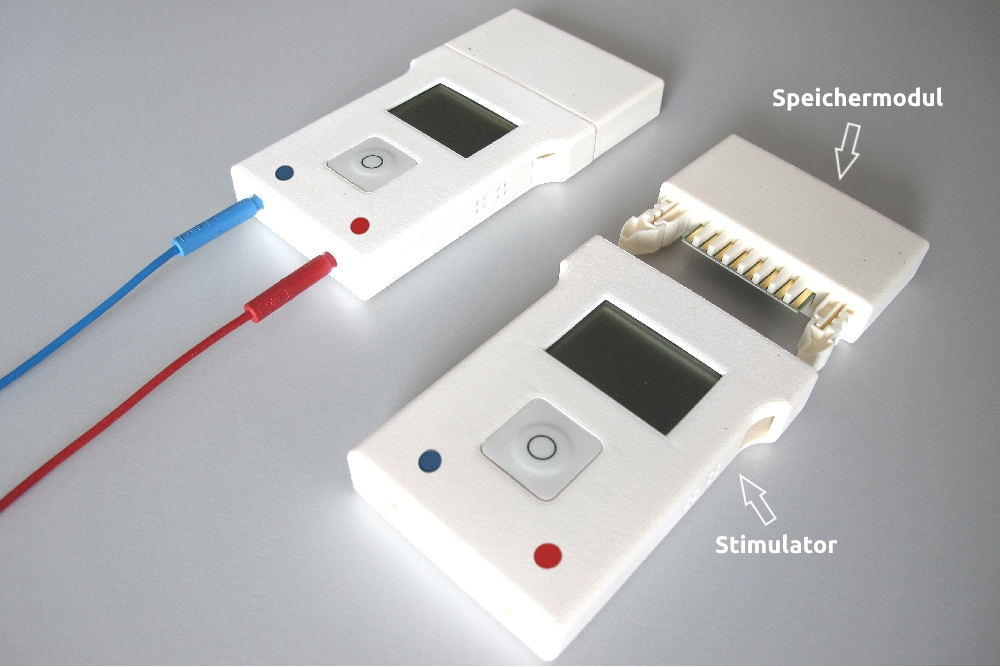
6 Storage modules
The storage module stores the parameters of the next stimulation (stimulation sequence(s), number and order of the enabled stimulation sequences and a start time, if desired), as well as the electrical energy necessary for the operation of the stimulator.
The log-data recorded during an ongoing stimulation is also stored in the storage module.
2 Stimulators
Enter the description for this row.
2 Programmers
The programmer transfers data between the PC software and the storage module and can also charge the battery contained in each storage module.
PC-software
To operate the DC-STIMULATOR MOBILE, a computer with a stable internet connection and a USB port is required. The software provided by neuroCare organises the configuration and charging of the storage modules, as well as the transfer of log-data into the databank.
Case
Each SOD is delivered in a hardshell case. This allows for the devices to be safely transported and conveniently stored.
Technical Specifications
Stimulation
- tDCS, DC intensity of -2,000 μA up to +2,000 μA,
- Deviation of the nominal value of DC current: max. 2 %
- Hardware offset: ±10 μA
- Voltage limit: max. ±16 V
Power consumption
Power Supply
Battery life
Dimensions
-
Stimulator: 71 mm x 94 mm x 15 mm, weight 66 g
-
Programmer: 71 mm x 62 mm x 15 mm, weight 46 g
-
Storage module: 71 mm x 39 mm x 15 mm, weight 42 g
-
Charge only device (optional): 71 mm x 61 mm x 15 mm, weight 46 g
Accessories for the DC-STIMULATOR MOBILE
The DC-STIMULATOR MOBILE is delivered with an equipment starter set, which contains a set of standard accessories: electrodes of various dimensions, cables, sponges and straps.
All components can be reordered from neuroCare. Please contact us if you wish to reorder complete sets or individual components: sales@neurocaregroup.com or 03677-689790.

Use in clinical trials
If you intend to use the DC-STIMULATOR MOBILE in double blind clinical studies applying transcranial electrical stimulation (tDCS, tACS, rTMS) please contact the manufacturer or responsible sales department.
In clinical research the devices are used by the German Center for Brainstimulation as well as the Charité in Berlin, the LMU in Munich, the University hospital Hamburg-Eppendorf in Germtes worldwide.

Product-specific downloads
Enquire today
We are here to help you find the right technology and training for your practice or research
French materials
Download our patient flyers in French language:
Flyer: tDCS - Thérapie efficace pour la douleur,
la dépression, les accidents vasculaires cérébraux
Flyer: tDCS - Traitement effectif pour
dépression
Flyer: tDCS - Thérapie combinée efficace
dans la réhabilitation
